Shares of Vertex (VRTX) declined 20% last week after the company announced the discontinuation of VX-814, the company’s candidate for alpha-1 antitrypsin deficiency. The decline seems disproportionate to the perceived value of this program, but it underscores Vertex’s main weakness – its relatively early-stage and unproven pipeline outside of cystic fibrosis (CF). The stock looks attractively valued today based on growth estimates, but there is a limit to value creation coming from the CF franchise, and the company should be more aggressive with pipeline building in the following quarters and years.
VX-814 was not worth anywhere near $13 billion, but the clinical setback has impacted investor perception of the company’s pipeline
Vertex’s valuation was cut by approximately $13 billion when the company announced the discontinuation of the VX-814 program for alpha-1 antitrypsin deficiency (AATD). The company was running a phase 2 trial to determine the safety and pharmacokinetics of VX-814, and it observed elevated liver enzymes in several patients. In four patients, across different doses, elevations greater than 8 times the upper limit of normal were noted, and the analysis of the PK data indicated that exposures achieved were too low and that it is not feasible to safely reach targeted exposure levels to meaningfully increase AAT levels (AAT is the deficient protein).
But all is not lost in AATD, as Vertex has a second candidate in development, VX-864, which is also in a phase 2, proof-of-concept study. The study was initiated in July 2020, and initial results are expected in 1H 2021.
There is no way to claim this program was valued anywhere near $13 billion (especially considering the lack of positive clinical proof-of-concept data), but the failure underscores the company’s main weakness – its reliance on the CF franchise and the lack of meaningful pipeline behind it.
CF franchise remains the key value driver, and the company should use increasing cash flows to expand the pipeline
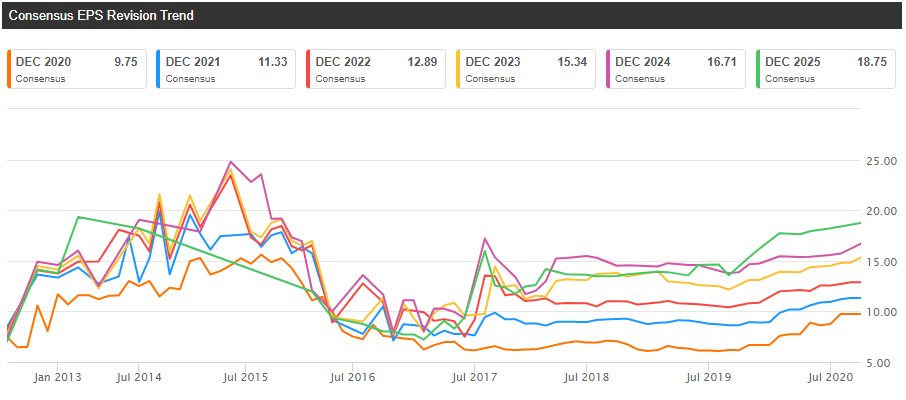
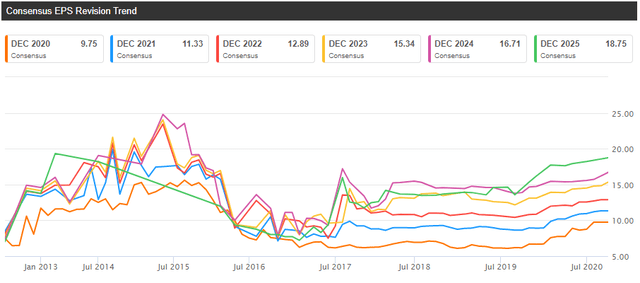
Vertex appears attractively valued based on its CF franchise alone. The stock is trading at approximately 20x next year’s EPS, which seems relatively low for a company delivering 30%+ revenue growth and one in the midst of significant margin expansion and earnings growth. And earnings and revenue revisions keep trending higher, which is what you want to see in a growth stock. As an example, the 2021 EPS consensus was $8.93 at the end of 2019 and it is $11.33 now. Similarly, the revenue consensus increased from $5.73 billion to $6.82 billion.
Source: Seeking Alpha
Estimates are going up due to the strong launch of Trikafta, the first triple combination therapy approved to treat patients with the most common CF mutation. Trikafta was approved in the U.S. in Q4 2019 for patients aged 12 and older, and it has already reached $918 million in net sales in the second quarter of 2020. And it is not even launched in Europe yet (it will be called Kaftrio in Europe). Approval in Europe is expected by year-end, and it should represent a nice tailwind for the company in 2021 and beyond.
Approvals for Trikafta/Kaftrio for younger patients should also lead to additional revenue growth in 2021 and beyond, starting with patients ages 6-11. The company says approximately 68,000 patients, or 90% of the population, should be eligible for treatment in the not-too-distant future.
Vertex’s dominance in CF has so far been unchallenged, but we should not assume its dominance will remain intact in the future. There are other programs in development that could be competitive or complementary to the company’s therapies.
Inhibition of the epithelium sodium channel (ENaC) is one interesting approach, and inhaled therapies are in development. Ionis (IONS) recently reported positive proof-of-concept results, although the results were in healthy volunteers, not CF patients. The 75mg dose of Ionis’ IONIS-ENAC-2.5Rx demonstrated a mean 55.6% decrease in ENaC mRNA expression in the multidose segment of the trial. In preclinical studies, ENaC reductions of 40% or more resulted in significant improvement in mouse models of CF lung disease.
However, even if the company’s dominance remains unchallenged for years to come, there isn’t too much room for value creation in the long run, as there are only so many CF patients to treat. That’s why Vertex is, and should be, investing heavily in pipeline expansion.
As mentioned, there is a second shot on goal in AATD with VX-864, and initial results are expected in 1H 2021.
The company is running a phase 2 study of VX-147, a small molecule inhibitor of APOL1 function – a causal genetic factor in focal segmental glomerulosclerosis (FSGS) – and if successful, it could be applicable to other proteinuric diseases. Proof-of-concept results are expected in 2021.
Vertex also has a gene-editing collaboration with CRISPR Therapeutics (CRSP) and one candidate (CTX-001) in the clinic for the treatment of beta-thalassemia and sickle cell disease (SCD). CTX-001 has achieved positive proof-of-concept results in both indications.
- Two transfusion-dependent beta-thalassemia patients have achieved high total hemoglobin and fetal hemoglobin levels. The first patient has now been transfusion-free for more than 14 months (versus 34 units per year prior to treatment) and the second patient 3.5 months (versus 61 units per year prior to treatment).
- One SCD patient was treated, and the patient demonstrated an increased level of total hemoglobin and fetal hemoglobin. The patient had zero vaso-occlusive crises (VOC) in 9 months of follow-up and no transfusions since day 19 following the infusion of CTX-001.
And though the data were reported in just three patients across the two indications, the beta-thalassemia study dosed a total of 5 patients and all patients have successfully engrafted, and the SCD study has dosed 2 patients and both patients have successfully engrafted. The initial safety profile in both trials appears consistent with myeloablative busulfan conditioning and an autologous hematopoietic stem cell transplant.
It is too early to count on CTX-001, as the results to date are only in 3 patients across two indications, but the initial results look promising.
The company also expects to submit an IND by year-end for a potentially curative cell-based treatment of type 1 diabetes.
The pipeline seems rather light for a $55 billion market cap company, and there are no candidates outside of CF that will be close to approval in the next 2-3 years.
The good news is that Vertex has ample resources to expand its pipeline and/or product portfolio. It ended Q2 with $5.5 billion in cash and equivalents, an increase of $1.7 billion from December 2019. Quarterly cash flow should further improve in the following quarters, and the company can also use equity or debt to boost its buying power.
Conclusion
Vertex is one of the strongest big-cap biotech growth stocks, thanks to the dominance of its CF franchise, which should continue to generate significant revenue growth and also strong cash flow growth in the following quarters and years. The stock also looks attractively valued relative to its growth profile, and it should continue to be a decent compounder in the following years.
However, I believe there is a limit to value creation coming from the CF franchise and that the next phase of value creation will likely come from the company’s expanding pipeline. And I do not think the existing pipeline is enough to get there (or at the very least, not in the next 2-3 years). There is an evident hole in the pipeline – there are no mid/late-stage candidates in the clinic – but the cash is there to be used to plug that hole, and I expect the company to use it in the following quarters.
I publish my best ideas and top coverage on the Growth Stock Forum. If you’re interested in finding great growth stocks, with a focus on biotech, consider signing up. We focus on attractive risk/reward situations and track each of our portfolio and watchlist stocks closely. To receive e-mail notifications for my public articles and blogs, please click the follow button. And to go deeper, sign up for a free trial to Growth Stock Forum.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Additional disclosure: This article reflects the author’s opinion and should not be regarded as a buy or sell recommendation or investment advice in any way.