After listening to Omeros’ (OMER) 10/22/20 conference call on the final data readout for treatment of hematopoietic stem cell transplant-associated thrombotic microangiopathy (HSCT-TMA), I am extremely sanguine about Omeros’ prospects. I see it as a prime investment opportunity which is underappreciated by the market.
This article explains my thinking.
Omeros expects to file its BLA for treatment of HSCT-TMA within a matter of weeks.
I first reported on my optimism surrounding Omeros’ narsoplimab in treatment of stem cell TMA in a 6/2018 article, “Omeros: Payoffs Beckon, Risks Abound.” At the time, having already achieved breakthrough and orphan drug designations, Omeros was in talks with the FDA about establishing a procedure for submitting a rolling BLA to the FDA.
In several articles since, I have chronicled Omeros’ laborious progress as it rolled its BLA towards the finish line, just last August noting:
If all goes well, Omeros will file its BLA in stem cell TMA by the close of this quarter; it will be accepted by the FDA within 60 days and approved for marketing six months thereafter. That would pave the way for a launch in mid-2021.
Discouragingly, Q3 2020 passed with no BLA filed. Yet now we are only a few weeks into Q4 2020 and all is looking good. No, the BLA is not yet filed, but its 10/22/20 results conference call (“RCC”) (free sign-up required) on final efficacy and safety data for narsoplimab in treatment of HSCT-TMA reported good news. The BLA is complete, undergoing a process of internal review, a publication phase as Omeros puts it, so that it will seamlessly integrate with the FDA systems for its review processes (32:30-34:45/1:04:53).
Omeros has not set a target date for actually submitting the BLA but has stated that it will be soon, likely in a matter of weeks, not months. Omeros is making the effort on the front end to dot the i’s and cross the t’s, so that it does not face a Refusal to File letter which can hamstring an otherwise promising therapy (40:46-43:11 /1:04:53).
At the moment, cynicism seems to be the controlling investor sentiment. Since the call, which I regarded as highly positive, the share price has been declining per its chart below from 10/22/20 to just before market close on 10/23/20 as I write:
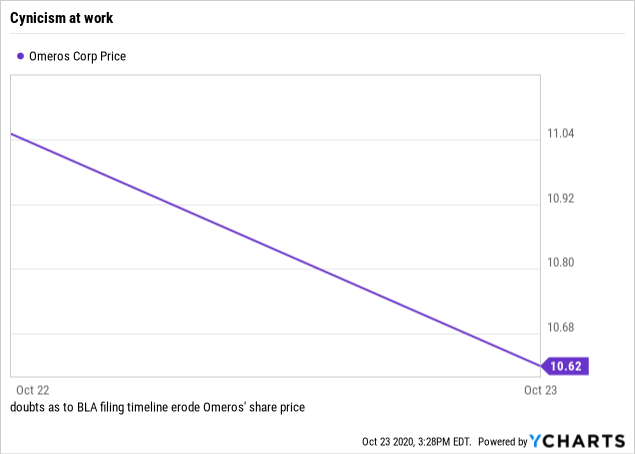 Data by YCharts
Data by YChartsI get it that shareholders are growing weary of the drawn-out BLA time frame. However, it seems a pity to throw up one’s hands at this point. Omeros is on the threshold of achieving a long awaited goal. The game is definitely worth the candle. With its paltry market cap of ~$0.65 billion (10/25/20), Omeros is a bargain.
We have no guidance on expected revenues from narsoplimab in treatment of stem cell TMA. Using back-of-envelope figures of ~2,000 cases of stem cell TMA in the US annually, each costing the health system several million, it seems plausible to figure that stem cell TMA offers returns approaching blockbuster potential.
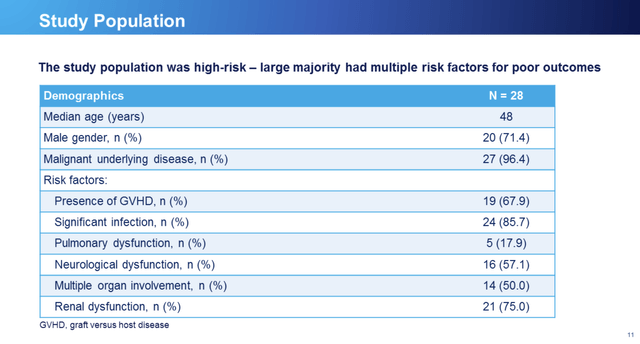
At the outset study participants were in parlous health per RCC slide 11 below:
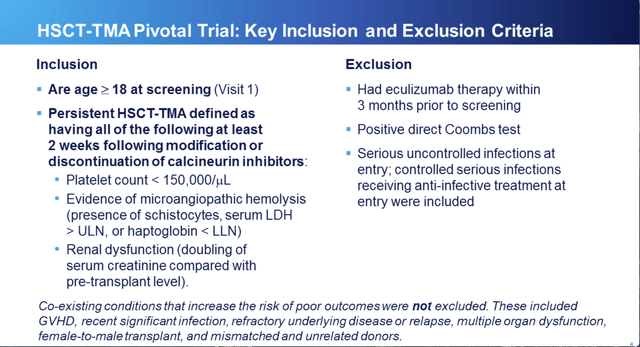
Study inclusion/exclusion criteria are set out at RCC slide 6 below:
Study objectives were made up of a composite of laboratory findings and clinical outcomes shown on RCC slide 7 below: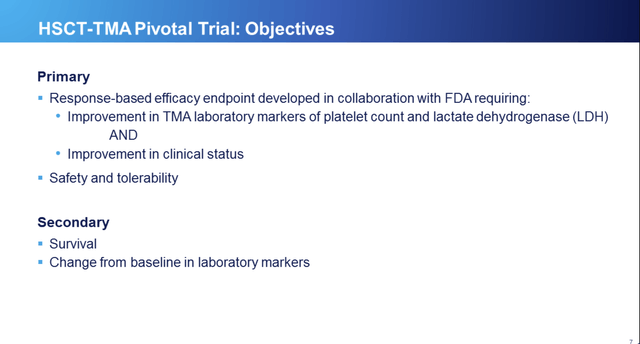
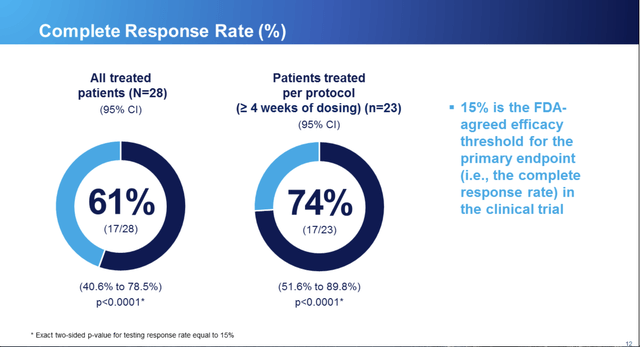
As for Omeros’ trial results, they blew away the primary endpoint established at the outset of the study with the FDA as shown by RCC slide 12 below:
Omeros’ current liquidity buffers appear adequate to see it through its narsoplimab launch, albeit additional dilution may become necessary.
Omeros’ one approved therapy, OMIDRIA used in cataract and other eye surgeries to maintain pupil size and to relieve pain, has been Omeros’ breadwinner over the years. Its 2020 10-K (p. 48) lists the following product sales for OMIDRIA for years 2015-2019:
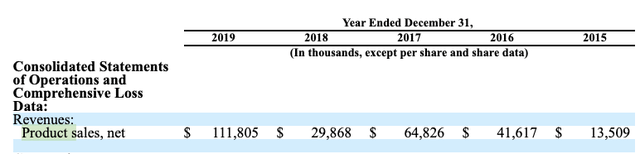 OMIDRIA’s crazy revenue trajectory, with product sales so truncated for 2018, is all about reimbursement. When supported with appropriate pass-through billing it has shown great promise. At the current time it both lacks this support and is under a cloud from the resurging pandemic.
OMIDRIA’s crazy revenue trajectory, with product sales so truncated for 2018, is all about reimbursement. When supported with appropriate pass-through billing it has shown great promise. At the current time it both lacks this support and is under a cloud from the resurging pandemic.
In its Q2, 2020 earnings press release, Omeros described the situation as follows:
For the second quarter of 2020, revenues, all related to sales of OMIDRIA, were $13.5 million, down from $26.8 million for the same period in 2019 and $23.5 million for the first quarter of 2020. The decrease in the current quarter is due to ambulatory centers (ASCS) and hospitals postponing nearly all cataract surgeries from mid-March until mid-May due to the COVID-19 pandemic. Cataract surgeries resumed beginning in the second half of May 2020 and by the end of June 2020, the run rate of weekly OMIDRIA sales approximated levels seen prior to the pandemic.
OMIDRIA’s likely product sales for Q3 2020 are a riddle. They will be positively impacted by relaxed COVID-19 shutdowns. On the negative side, OMIDRIA’s two-year pass-through extension expired 10/1/20. In this respect Q3 2020 is analogous to Q4 2017, insofar as both are quarters immediately preceding episodes of expiration of pass-through status for OMIDRIA. If such analogy holds, Q3 2020 OMIDRIA sales will be adversely impacted by pending reimbursement uncertainty as was the case in Q4 2017.
Omeros reported a slim balance of $16.1 million in cash, equivalents and short-term investments in its Q2 2020 financial press release. Such a sum is clearly inadequate for a company with uncertain revenues which has quarterly expenses running well >$35 million a quarter.
By my lights, Omeros has operated with inadequate liquidity buffers as long as I have been following the stock. Its Q2 2020 balances nonetheless were extreme. It redeemed itself as a financially viable operation with its concurrently announced financings of $125 million stock and $200 million of convertible senior notes.
Omeros’ exact cash runway will be unclear until it reports its Q3 2020 earnings. Variables include its ongoing expenses, its OMIDRIA revenues and how much of the $200 million note financing it nets after repayment of earlier borrowing.
Even with minimal ongoing contribution from OMIDRIA, Omeros has sufficient liquidity without need for additional dilution to take it to its HSCT-TMA decision date.
Narsoplimab’s stellar impact in its few uses as a therapy for COVID-19 provides a potential bonus for Omeros investors.
When I first learned of Omeros’ success in treating the six compassionate use patients in Italy, I was gratified but dismissive. There is so much noise surrounding potential COVID-19 therapies that it is difficult to get excited about a therapy that has yet to even enter the lists.
It’s a pity that Omeros is still at such an early phase of its corporate development. Narsoplimab as a therapeutic for COVID-19 has some significant attractions. During Omeros’ 10/22/20 RCC, we learned some interesting tidbits.
In response to follow-up questions it turns out that COVID-19 patients who were treated with narsoplimab have progressed admirably in subsequent testing. Not only are their clinical outcomes free of sequalae which so often dog COVID-19 patients, but also their laboratory findings are, at 5-6 months follow-up, “entirely normal” (39:00-39:40 / 1:04:53).
As I discuss in some detail in “Omeros’ COVID-19 Opportunity And Challenges,” COVID-19 will be a tough nut for Omeros to crack should it ever make the effort. Nonetheless, it will be a shame if a therapy which shows such promise is never developed.
I come at the issue with the expectation that narsoplimab will merit FDA approval in stem cell TMA. Should it do so there is ample reason to expect it to also have impact in treatment of COVID-19 as reflected by journal articles such as “Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications” and “Differences and similarities between disseminated intravascular coagulation and thrombotic microangiopathy.”
Omeros currently lacks the resources to effectively advance narsoplimab as a COVID-19 therapy. To the extent of the credible evidence of narsoplimab’s potential in this area, it should attract grants and potential for deals. To this point, COVID-19 has shown that it has an unfortunate staying power. Even when an effective vaccine arrives to minimize its power, there will still be a need for effective therapeutics.
Omeros presents a substantially derisked opportunity to investors with a high reward potential.
Omeros has greatly derisked its upcoming rounds with the FDA as it seeks approval for narsoplimab in treatment of HSCT-TMA as discussed above. Crippling delays in the form of a refusal to file letter or a complete response letter as its PDUFA date nears are nonetheless lurking possibilities.
As I have followed its progress, I am optimistic that Omeros will dodge those bullets and will achieve FDA approval for narsoplimab in this indication by Q3 2021. Assuming it does so it will enter a new phase, another danger zone as it works to successfully commercialize narsoplimab.
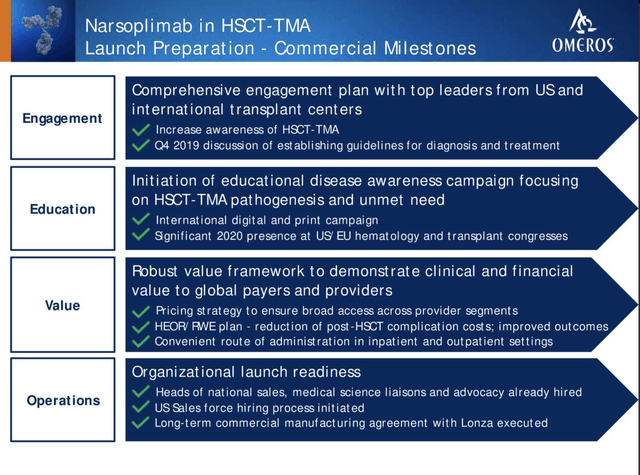
Omeros has shared its launch preparations in some detail as reflected below from its 6/20 investor conference slide 21:
While Omeros has shared lots of management commentary on its upcoming FDA submittal and its launch preparations, we have very little on sales projections. During its Q1 2020 earnings call CEO Demopulos did note:
We’ve developed the narsoplimab value story and are conducting market research with transplant physicians, administrators and payers. We’re finalizing our distribution and pricing strategies as well as our profiling activities to ensure a well designed and executed launch for narsoplimab and transplant TMA.
OMIDRIA, Omeros’ long-term value driver, is re-entering a period of uncertainty; nonetheless, it will continue to generate sales during upcoming months and years. The amount of such annual sales may be in the millions of dollars, or should billing protection return, upwards of a hundred million dollars.
Omeros’ pipeline beneath OMIDRIA is broad and deep as shown below:
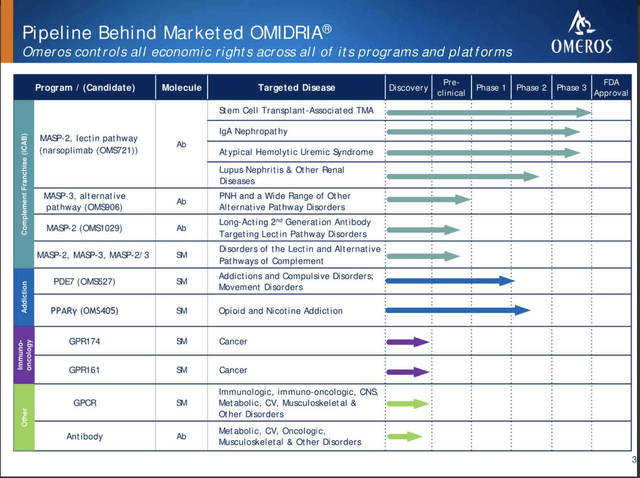 I discuss its development prospects at length in “Omeros‘ Deck Full Of Aces.”
I discuss its development prospects at length in “Omeros‘ Deck Full Of Aces.”
Conclusion
In sum, Omeros is a small biotech having <$1 billion market cap with a big future. Its prospects going forward are exceptionally appealing as it gets ready to present narsoplimab to the FDA for approval.
Omeros has been a challenging company for its investors over the years. At times, its OMIDRIA looked a solid bet to support ongoing clinical development of its pipeline. At others, not so much.
The situation as I write on 10/25/20 is an interesting one. OMIDRIA is at maximal uncertainty while narsoplimab is looking stronger than ever. Stronger for sure, but with hurdles yet to clear including:
- BLA filing [weeks];
- FDA approval of BLA with establishment of PDUFA date [60 days];
- FDA approval for marketing narsoplimab for HSCT-TMA [6 months];
- launch followed by successful commercialization.
Based on its RCC as discussed in this article, I am giving Omeros a high probability of reaching its launch hurdle by Q3 2021. At that point the situation moves to a sphere of high uncertainty. The task of estimating peak sales for narsoplimab in treatment of HSCT-TMA will no doubt prove contentious.
Consider that PubMed.gov includes an article abstract opening as follows:
Each year in the US, more than 10 000 patients benefit from allogeneic haematopoietic stem cell transplantation (HSCT), a modality that offers an excellent chance of eradicating malignancy but confers a higher risk of treatment-related mortality. An uncommon but devastating consequence of HSCT is transplantation-associated thrombotic microangiopathy (TA-TMA). The incidence of TA-TMA ranges from 0.5% to 76%, with a mortality rate of 60-90% despite treatment. …
Narsoplimab restores the majority of patients who respond to the condition that would have prevailed absent the transplantation-associated thrombotic microangiopathy (46:25-47:15 / 1:04:53). Eculizumab, another complement inhibitor used to treat HSCT-TMA, according to the RCC, is unsatisfactory in adult patients because of the high rate of complications and mortality (25:00 -26:07 / 1:04:53).
Eculizumab, brand name Soliris, is notorious as one of the highest priced drugs with a one-year course of treatment costing ~$500,000. This is in an arena which could produce peak sales consistent with my back-of-envelope estimate noted earlier.
Disclosure: I am/we are long OMER. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Additional disclosure: I may buy or sell shares in Omeros over the next 72 hours.